Lipophilicity: Understanding its Significance and Measurement Techniques
Lipophilicity is an important concept in the field of pharmacology and medicinal chemistry. It refers to the tendency of a molecule to dissolve or be absorbed in lipids or fats. This property plays a significant role in drug development and formulation, as it affects the drug’s bioavailability and pharmacokinetics.
In drug discovery, lipophilicity is a crucial parameter that needs to be optimized to ensure that the drug is effective and well-tolerated. A drug that is too hydrophilic or water-soluble may not be able to penetrate cell membranes and reach its target site, while a drug that is too lipophilic may be too readily absorbed by cell membranes, leading to toxicity or inadequate distribution to the desired site.
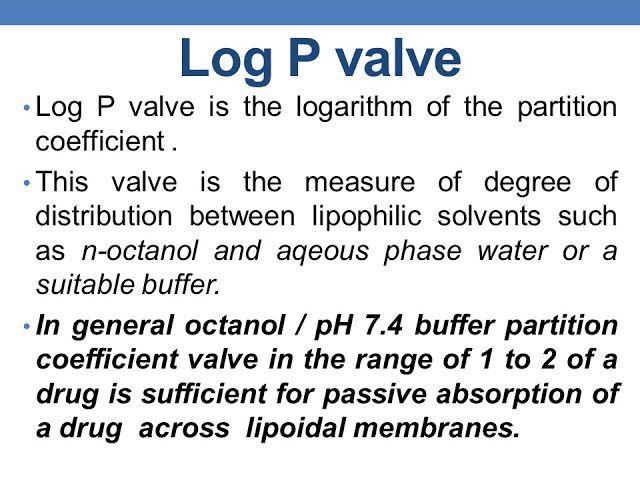
One of the primary ways to measure lipophilicity is through the use of partition coefficients. The most commonly used partition coefficient is the octanol-water partition coefficient (Log P). This measures the ratio of a compound’s concentration in octanol (a lipid-soluble solvent) to its concentration in water. A Log P value greater than zero indicates that the compound is more lipid-soluble, while a value less than zero indicates that it is more water-soluble.
Another measure of lipophilicity is the related concept of Log D, which takes into account the pH of the solution being measured. Log D values are particularly important for drugs that are weak acids or bases, as their solubility and partition coefficients can vary depending on the pH of the environment.
To optimize lipophilicity in drug discovery, medicinal chemists can adjust the molecular structure of the compound. This can be done through the addition or removal of polar groups, changing the position of functional groups, or adjusting the size and shape of the molecule.
In addition to drug development, lipophilicity is also important in cosmetic formulation. Many skincare products, such as moisturizers and serums, rely on lipid-soluble ingredients to penetrate the skin and deliver their active ingredients. For example, retinoids, a class of compounds derived from vitamin A, are highly lipophilic and are used in many anti-aging products.
To improve the delivery and absorption of lipophilic skincare ingredients, formulators may use emulsifiers or penetration enhancers. These help to solubilize the lipid-soluble ingredients and enable them to penetrate the skin more easily.
Overall, lipophilicity is a crucial property in drug development and cosmetic formulation. By understanding and optimizing this parameter, researchers and formulators can create safe and effective products that deliver their intended benefits.
Most searched products:
Does Sephora Support Israel? Answering Your Questions
2021’s Ultimate Guide to VIT Direct Reviews You Can’t Miss!
The Explosive Reaction: Sodium Hydroxide and Hydrochloric Acid
Benefits of Using Glycolic Acid for Dandruff Treatment
Capsaicin Cream Boots
Get Thick & Luscious Lashes With Sins N Lashes Serum: Our Honest Review!
The Ordinary Salicylic Acid
The Ultimate Guide to The Ordinary Alpha Arbutin 2% + HA for Flawless Skin
Exploring the Effectiveness of Ordinary Retinol for Skin: Is It Good?
Ultimate Guide to Tetrahexyldecyl Ascorbate: Benefits, Uses, and Side Effects