The Ultimate Guide to Understanding HCL Molecular Weight
Molecular weight is a crucial factor in chemistry that determines the physical and chemical properties of different elements and compounds. HCL, also known as hydrochloric acid, is a colorless and highly corrosive solution that is widely used in various industries. The HCL molecular weight plays a critical role in determining the concentration, molarity, and reactions of the acid. In this article, we will discuss the importance of the HCL molecular weight and its relevance to industries such as medicine, agriculture, and manufacturing.
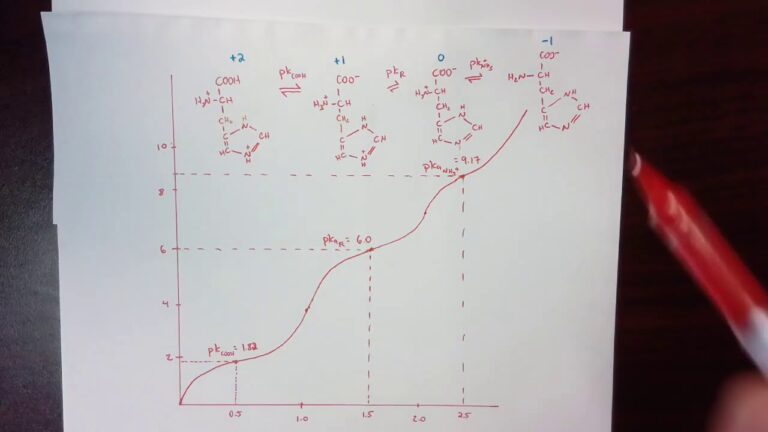
What is the Molecular Weight of HCL?
The molecular weight of HCL is the sum of the atomic weights of hydrogen (H) and chlorine (Cl) present in the compound. The atomic weight of hydrogen is 1.00794 and chlorine is 35.453. Therefore, the molecular weight of HCL can be calculated as follows:
Molecular weight of HCL = Atomic weight of H + Atomic weight of Cl
Molecular weight of HCL = 1.00794 + 35.453
Molecular weight of HCL = 36.46094
This means that the molecular weight of HCL is approximately 36.5 grams per mole (g/mol). This information is vital in determining the amount of HCL solution needed for specific reactions in different industries.
Importance of HCL Molecular Weight in Medicine
HCL is widely used in the production of different medications due to its acidic properties. However, the concentration of HCL in each medicine varies depending on the reaction requirements. The HCL molecular weight plays a crucial role in determining the concentration of the acid required for specific medication batches. For instance,
Pepsinogen to pepsin conversion in gastric juice needs hydrochloric acid, a reaction that requires the right concentration of HCL with the ideal molecular weight.
Importance of HCL Molecular Weight in Agriculture
Hydrochloric acid is a vital compound in agriculture, primarily in the production of fertilizers. Fertilizers such as ammonium chloride, calcium chloride, and ferrous chloride are widely used in agriculture to improve crop productivity. These fertilizer compounds are made possible due to the molecular weight of HCL. The ideal HCL molecular weight creates the right concentrations required for the production of different fertilizer grades. For instance, calcium chloride with a molecular weight of 111 g/mol is vital in correcting calcium deficiency in plants.
Importance of HCL Molecular Weight in Manufacturing
HCL is also widely used in the manufacturing industry for various processes such as food processing, metal cleaning, and water treatment. The HCL molecular weight determines the concentration and reactivity of the acid, making it crucial in various manufacturing processes. For instance, in metal cleaning, the ideal HCL molecular weight ensures that the right concentration is created for efficient rust removal from metal surfaces.
Conclusion
The HCL molecular weight is a crucial factor in different industries such as medicine, agriculture, and manufacturing. The molecular weight helps in determining the right concentration, reactivity, and compounds that can be created from the compound. Therefore, having a comprehensive understanding of the HCL molecular weight is vital in ensuring efficient and safe production in different industries.
Contents
Most searched products:
Does Sephora Support Israel? Answering Your Questions
Get Smoother Feet with Glycolic Acid: The Ultimate Guide
The Ordinary Foundation Colour Match
10 Proven Skincare Products for Effective Treatment of Seborrheic Dermatitis
Benefits of Using Glycolic Acid for Dandruff Treatment
The Complete Guide to The Ordinary Hyperpigmentation: Causes, Treatment and Prevention
The Ultimate Guide to The Ordinary Squalane Cleanser for Clear and Nourished Skin
Liaison Lash Serum: The Ultimate Solution for Longer and Fuller Lashes
Lumin Skin Care Reviews: The Ultimate Guide to Glowing Skin
All You Need to Know About Tretinoin: The Ordinary’s Best Skincare Solution