The Ultimate Guide to Zinc and Hydrochloric Acid Reaction
Zinc and Hydrochloric Acid: A Powerful Reaction
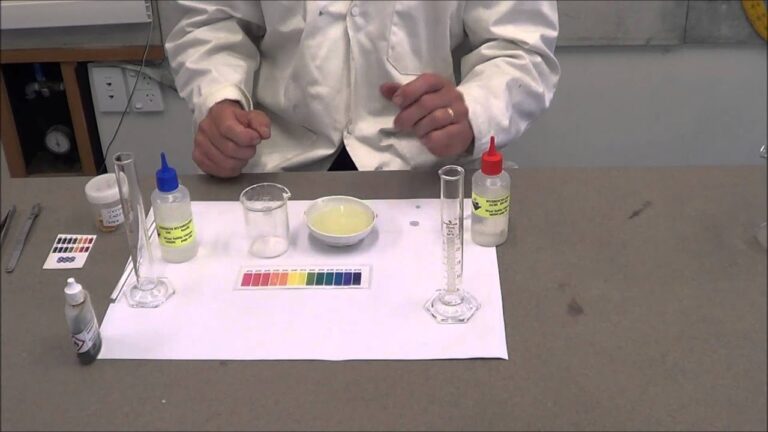
When you think of chemistry experiments, the image of bright-colored liquids bubbling away in a test tube may come to mind. One of the most stunning reactions that you may witness in a chemistry lab involves the mixture of zinc and hydrochloric acid. When combined, these two substances produce a reaction that releases hydrogen gas and turns the zinc into a soluble form. So why is this reaction so powerful, and how does it occur? Let’s explore this topic further below.
What is Zinc?
Zinc is a metallic chemical element that is commonly found in the Earth’s crust. It has the atomic number 30 and is symbolized by the letter Zn. Zinc is a bluish-gray metal that is brittle at room temperature and can easily be molded or shaped. This metal is well-known for its anti-corrosive properties, which makes it a popular choice for coatings on other metals.
What is Hydrochloric Acid?
Hydrochloric acid is a strong, colorless acid that is commonly used in industrial settings. Its chemical formula is HCl, and it is composed of one hydrogen atom and one chlorine atom. Hydrochloric acid is highly corrosive and can dissolve many different materials, including metals and plastics.
The Reaction Between Zinc and Hydrochloric Acid
When zinc and hydrochloric acid are combined, a reaction occurs that results in the production of hydrogen gas and soluble zinc chloride. The chemical equation for this reaction is as follows:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
In this equation, the (s) and (aq) notations indicate the physical states of the reactants and products. The (s) stands for solid, while (aq) stands for aqueous, or dissolved in water.
Zinc reacts with hydrochloric acid to produce zinc chloride, which is soluble in water. The hydrogen gas that is produced during the reaction can be collected and measured, and a calorimeter can be used to determine the amount of heat that is released during the reaction.
Why is the Reaction Between Zinc and Hydrochloric Acid Important?
The reaction between zinc and hydrochloric acid is important for several reasons. First, it is a simple demonstration of a chemical reaction that produces visual and measurable results. Second, it is important in the field of metallurgy, as it can be used to determine the quality and purity of zinc-containing metals. Finally, the reaction is important in the production of various zinc compounds that are used in a wide range of industrial applications.
Conclusion
In conclusion, the reaction between zinc and hydrochloric acid is a powerful one that produces measurable and visual results. This reaction is used in a variety of applications, including metallurgy and the production of zinc compounds. Understanding this reaction can help scientists and engineers create new materials and products that are more efficient and effective.
Most searched products:
How Long Should You Leave on The Ordinary AHA BHA? The Ultimate Guide
The Ultimate Guide to Azealic Acid: Benefits, Uses, and Side Effects
Get brighter and smoother underarms with the original underarm brightening cream
Argireline vs Retinol: Which should come first in your skincare routine?
Discover the Best Way to Wear Boots with The Ordinary Foundation
How to Find Your Perfect Shade: The Ordinary Foundation Color Match Guide
The Ultimate Guide to Retinol: Benefits, Side Effects, and How to Use It
Breaking Down the Hype: Is The Ordinary Skincare Line Really Good?
Top 10 Salicylic Acid Products on Amazon for Clearer Skin
Unlocking the Benefits of Skincyclopedia Serum: Your Ultimate Guide